Abstract
Background: Allogenic hematopoietic stem cell transplantation (alloHSCT) is perhaps the only curative treatment for several haematological disorders. AlloHSCT is often complicated by acute graft versus host disease (aGvHD), the leading cause of transplant related mortality and morbidity. Steroids remain the first line standard of care for patients with GvHD. Unfortunately, 35-50% aGvHD patients become steroid refractory. There is paucity of drugs that are effective against steroid refractory GVHD. Therefore, there is a need to develop novel drugs for the treatment of GvHD. Withaferin-A (WA), a potent immunomodulatory and anti-inflammatory agent obtained from Withania somnifera, was investigated for this purpose.
Methods: To evaluate the in-vivo anti-GvHD potential of WA, a complete mismatch model of aGvHD was developed. Recipient BALB/c (H-2Kd) mice were given a myeloablative 6.5 Gy of total body radiation. The irradiated mice were transplanted with 5x106 bone marrow cells and 15x106 spleen cells from donor C57BL/6 (H-2Kb) mice resulting in aGvHD. Following transplantation, mice were divided into GvHD control and WA treatment groups. The WA treatment group was further divided into 4 sub-groups based on the clinical score (CS) or severity of GvHD at which treatment with WA was initiated i.e., CS 1-3, CS 4-6, CS 7-9, CS 9-11. WA was administered orally at a dose of 1 mg/kg for 21 days or till death of the animal, whichever was early.
To understand the mechanism of action, whole blood from healthy human volunteers were collected and human peripheral blood mononuclear cells (hPBMCs) were isolated using ficoll gradient method. hPBMCs were treated with WA (1µM) or dimethyl sulfoxide (vehicle control) for 2 hrs and cells were stimulated with PHA for the next 72 hrs. Media supernatant of all samples were collected for cytokine analysis using cytometric bead array and cells were processed for evaluation of proliferation and immune cell phenotyping by flow cytometry. JAK2-STAT3 signalling in hPBMCs was investigated using western blotting. These studies were initiated after clearance from institutional animal and human ethics committees.
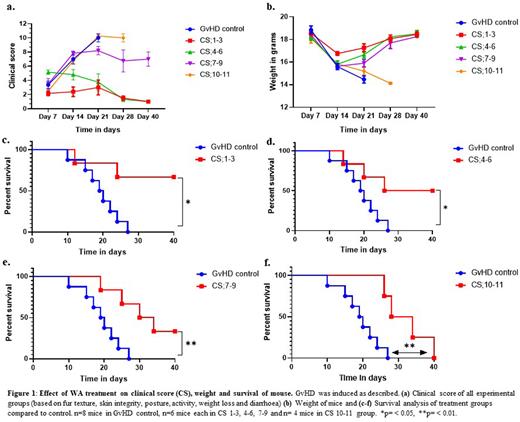
Results: WA treatment significantly improved the CS of aGvHD in terms of diarrhoea, weight loss, ruffling of skin and loss of movement (ANOVA for trend, P=0.02) (figure 1a-b). WA also significantly improved the overall survival irrespective of the severity of GvHD at the time of treatment (figure 1c-f). Proportion of animals surviving at day 40 was 0/8, 4/6. 3/6, 2/6 and 0/4 in GvHD control, CS 1-3, CS 4-6, CS 7-9 and CS 10-11 groups respectively (figure 1c-f). The hazard ratio in the four treatment groups were 0.19 (95% CI = 0.05-0.7), 0.29 (95% CI= 0.08-0.96), 0.25 (95% CI= 0.07-0.8), 0.23 (95% CI= 0.08-0.87) for CS1-3, CS4-6, CS7-9 and CS10-11 respectively in comparison with GvHD control.
In the ex-vivo treated hPBMCs, the proliferation of T-cell subsets (CD3+, CD4+ and CD8+ cells) significantly decreased in the presence of WA compared to control (P=0.002, 0.008, 0.009 respectively). Similarly, the immune subsets of absolute monocyte, classical monocyte, PD1-CD4 cells and Tim3-CD8 cells were found to be significantly decreased on treatment with WA. In contrast, the absolute non-classical monocytes and γδT-cells increased in the presence of WA. Further, levels of cytokines such as IFN-γ, IL-6, TNF-α, IP-10, IFN-L1, GM-CSF, IL-1β and IL-10 were found to be significantly decreased (P=0.008, 0.01, 0.01, 0.01, 0.003, 0.04, 0.02 and 0.01 respectively) by WA with respect to control. We also observed significant inhibition of phospho-JAK2 and phospho-STAT3 proteins in WA treated cells (P=0.002 and 0.03 respectively). However, total protein levels of JAK2 and STAT3 remain unchanged between control and WA treated cells.
Conclusion: WA abrogates GvHD and improves survival by regulating immune cell proliferation, differentiation and cytokine storm via inhibition of JAK2-STAT3 signalling. Survival data from mice supports further investigation of WA for GvHD in a prospective clinical trial.
Disclosures
Hingorani:Pharmza Herbal Pvt. Ltd.: Current Employment, Other: Current holder of individual stocks in a private limited company.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal